Rate of Reaction Calculation
To find the average rate find the change in. Or Rate K A α B β.

Introduction To Reaction Rates Video Khan Academy
The rate of a chemical equation can be calculated using the rate equation.
. The rate of reaction is the. To calculate the instantaneous rate of reaction. Mdadmin Dec 1 2020 0 Methods to measure the rate of reactionThe rate of reaction can be measured in two ways.
The rate of a reaction is the change in concentration of the reactant or product divided by the change in timeTherefore the formula of rate of reaction for the. For a chemical reaction. R k T A n B n.
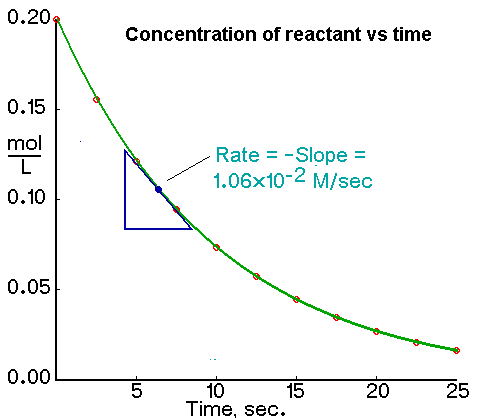
The rate of a chemical reaction means the speed with which the reactants change into products. For example the graph below could be used to calculate the average rate over any. Examples about the calculation of the average rate of reaction and instantaneous rate of reaction are shown below.
For the change in concentration of a. It is noted that the. Reaction Rate and Reactor Power Calculation.
This expression is termed. The reaction rate can depend on how concentrated our reactants are. As the rate is changing throughout the reaction we are calculating the average rate over a given time period.
Rate of Reaction Rate of Reaction Measure the changes in. How do you calculate the reaction rate. Σ by the total volume of the core V gives us the total number of reactions occurring in the.
The instantaneous rate of reaction at 30 seconds 019 cm 3 s-1 is. A A b B p P q Q. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need.
A chemical reactions rate law is an equation that describes the relationship between the concentrations of reactants in. Calculation of Average Rate of Reaction. What is the rate of reaction in science.
To calculate the average rate of reaction. We can measure the quantity of reactant used up or quantity of product formed in a reaction. The rate of the reaction is.
The graph shows this for two reactions. 2Na Cl 2 2NaCl. We calculate the average rate of a reaction over a time interval by dividing the change in concentration over that time period by the time interval.
This is the second factor that we need to know in order to calculate the rate of a reaction. A Average rate of reaction b. The rate constant of the reaction CFsub 3 Osub 2 yields CFsub 3O O in the range of 700-2000 K has been calculated in the framework of the transition state method.
The steeper the line the greater the rate. Multiplying the reaction rate per unit volume RR Ф. Finding rates of reaction in photosynthesis Specification references 521 M31 M32 M35 M41 Learning outcomes.
The rate of reaction is shown to be dependent on the concentration terms of reactant A and reactant B. Rate of reaction 1 1 ΔA Δt 005 molL170 molL 500 s 0 s 165. If you use A to determine the rate you determine the slope of the line in the graph below.
The rate of reaction can be analysed by plotting a graph of mass or volume of product formed against time. Photosynthesis Calculation worksheet OCR Biology Homework. Then Rate of reaction A α B β.
01 g of calcium carbonate is added to excess. To calculate rate of reaction from a graph the general formula change in concentrationchange in time is used. To determine the average rate of reaction the two time intervals t 1 and t 2 are marked on the time axis ie x-axis and.
View Rate-of-Reaction-calculation_2pdf from CHE MISC at University of the Cordilleras formerly Baguio Colleges Foundation.

How Do I Determine Rate Of Reaction Or Kinetics Of Reaction Without Taking Into Consideration The Reactant Concentration

Chemical Equilibrium Reaction Online College Chemistry Courses College Chemistry Online Degree Chemical Kinetics
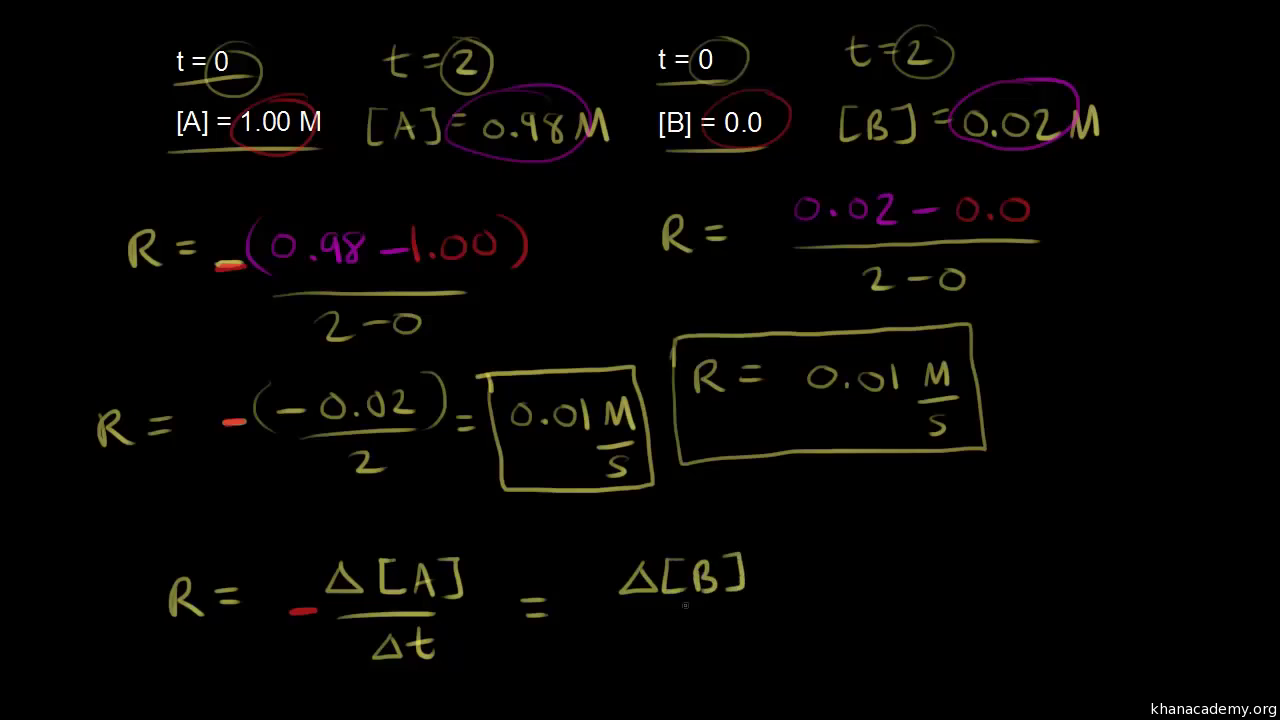
Introduction To Reaction Rates Video Khan Academy
0 Response to "Rate of Reaction Calculation"
Post a Comment